
Implementation of Engineered Infection Control Solutions in a Food Production Factory.
Introduction
PP-L Biosafety were commissioned to provide engineered infection intervention solutions for one of the Europe’s largest food manufacturers across several of their production sites in the UK. This was instigated after a site in Scotland became problematic for the client due to a COVID outbreak and the business disruption of a necessary shutdown, sending hundreds of workers home.
At the instigation of the Client and Regulators, PP-L were engaged to engineer infection mitigation solutions for the factories, in conjunction with an infection risk audit, and validation of the solutions, conducted by an independent laboratory to validate the efficacy of the solution’s specified.
The first study was undertaken by Micro-search Laboratories, with the goal of understanding the effect that PP-L’s ultraviolet based germicidal filters will have for these large industrial applications by assessing against a comparable but safe COVID-19 substitute, a similar airborne RNA virus, PHI 6. Tests were undertaken by sampling viral concentrations at several intervals over a 60-minute period. The results were incredible.
Factory environments can present an ideal microbial breading ground, especially in the food sector but now, given the airborne viral hazard that causes Covid-19, even more so. Coronavirus is at optimal infectivity within 5-14 degrees C range which are typical for food manufacture and chilled storage temperature ranges but worse, the air systems in food factories are usually recirculated by the chiller units that are essential to keep the environment cool and food-safe and so, represent the very highest-risk environments for the potential transmission coronavirus in a workplace setting according to statistics

As well the environmental setting, the site in Scotland was also facing the other common problems that the whole food manufacturing sector faces:- the risk of close proximity between staff members working on the lines and also, people shouting over the noise of machinery; shift change-overs create overcrowding; pinch points also can lead to accelerated transmission from the higher viral loads generated.

These risks demonstrate the need for engineered infection control solutions to be integrated into workplaces, mitigating the risks that the food and manufacturing sector struggles to avoid by solely following the guidance alone. The risk can be managed and importantly, business operations and production can continue at desired rates, even more safely, almost normally. The HSE general guidance is important but, the risk assessment of the workplace’s such as these, one must inevitably include the need for infection control engineering to mitigate hazards to suit the given environments and operations as are appropriate on a case by case basis.
PP-L’s design process incorporates assessments of specific workplace risk, observing the occupancy within given spaces, understanding potential bio-load concentrations and pinch points. It is also crucially important to model the humidity, temperature, ventilation air flow, filtration, and air distribution within the infection control process. From these parameters, one can identify the ventilation improvements needed inside buildings but importantly for the highest risk environments, it is probable that one must deploy germicidal UV Filters to improve the ventilation because the alternative, supported by the HSE, HEPA Filters, are inappropriate in these settings.
One must calculate the correct UVC dose, select and specify the correct devices and their position, so that one can safely inactivate the target microbes at source but also as part of the holistic primary building HVAC system. Moisture, temperature and air cooling effects must also be considered and in Food Factories, one must recognise the regulations and HACCP issues around glass and food safety. Special tried, tested and proven precautions all need to be considered with respect to UV lamp sepcifications.
PP-L concluded that the engineered infection control solution for the factory in Scotland was the installation of a mixture of different types of active, fan assisted, UVC air disinfection devices to suit the various environmental conditions in the factory, the room sizes, occupancy, viral load, operational and occupational risks. These units were installed by across variety of high-risk areas in the offices, factory, production plant, toilets, locker rooms, and corridors. Even the simplest devices, the upper room UV-C emitters, are a highly effective method for combating COVID-19 (Source) 2
Independent Laboratory Study
Micro-search Laboratories were commissioned by the Client and regulators to investigate the efficiency of specific PPL solutions. The efficiency of deactivating a COVID-19 substitute over a 60-minute period, compared with an independent condition with no UV-C solution present.
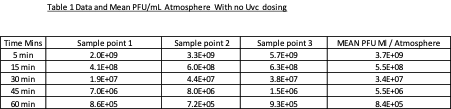

“Our data for stage 1 (no UV-c dosing) indicates that the initial Viral loading after the viral dispersion phase was at the Level of Log 9 plaque forming units per ml of atmosphere.
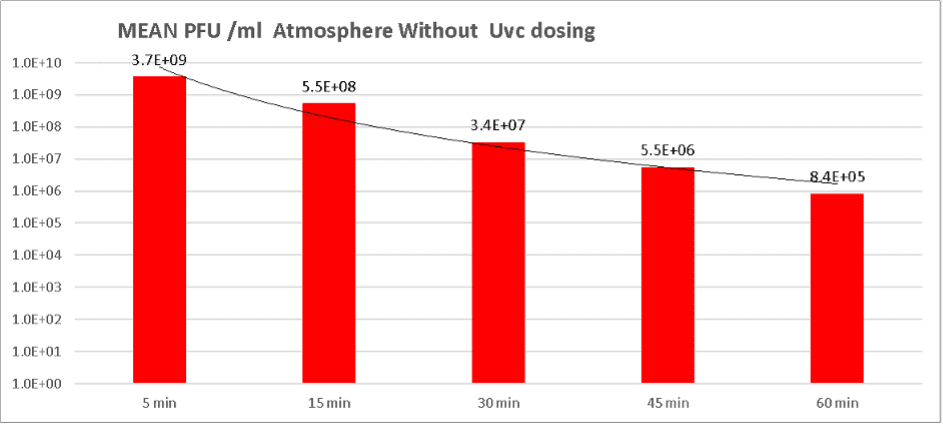
When sampling ended after a total 60 minutes the level had fallen to Log 5 plaque forming units per ml of atmosphere.
Our data therefore indicates that in stage 1 of the trial a 3.6 log reduction of atmospheric levels of viral particles occurred in the absence of UV-c. This is attributable and predictable due to the effect of a variety of physical forces.
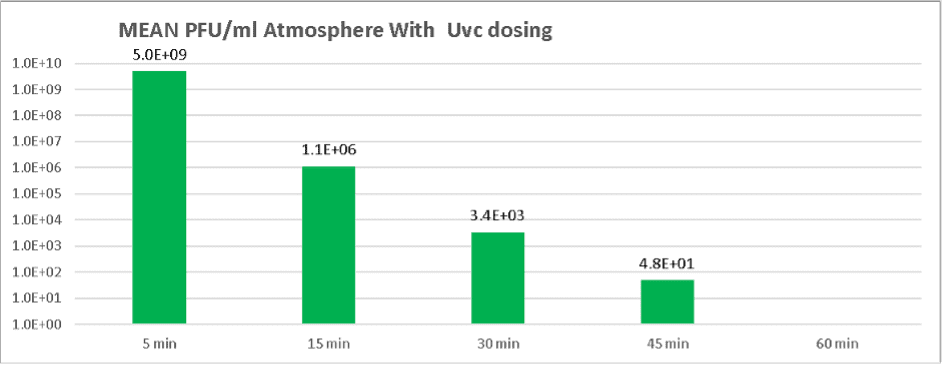
With UV-c dosing under similar conditions a much steeper rate of viral non recoverability was observed, with the initial mean challenge level falling from 5.0e9 PFU /ml to 4.8e1 PFU/ml by the 45-minute marker.
We could not recover any viable viral particles at the 60-minute point but the data at the 45-minute mark indicates at least an 8 Log reduction.
This result is > 5 Logs more lethality than was obtained in the same period with no UV-c dosing over the same period. It should be noted that the surrogate organism, the Phi6 phage is a double stranded RNA virus and likely to be 2-3 times more resistant to UV-c than the single stranded COVID 19
No official guidelines currently exist for the magnitude of viral reduction required to render atmospheres as safe so there is no current specific benchmark for Log reduction efficiencies. BS EN standards for other chemical based antimicrobial strategies generally specify a minimum of a 5 Log lethality. Clearly this device delivers performance which exceeds this level of expectation when run for 40 minutes after contamination of the test atmosphere.”
In layman’s terms, rather than the LOG or Lethal Dose terminology, the device’s performance under test in the UK confirms that it can disinfect the air better than most high street hand sanitisers by a factor of times x 10,000 !
Field tests in the factory confirmed performance of PP-L Biosafety’s solutions with statistical results demonstrating p<0.005 and extremely clean air against all the usual microbes commonly measured within the factories.
What is GUV or UVGI?
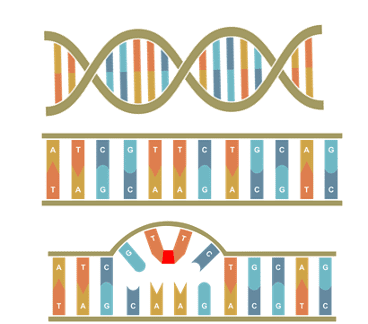 UV-C light is also known as germicidal light as it renders the DNA and RNA of microbes inactive by breaking bonds between the Thymine and Adenine pair and “glues” two adjacent Thymine nucleotides together. This process is irreversible and stops the microbes from undergoing mitosis and causing harm.
UV-C light is also known as germicidal light as it renders the DNA and RNA of microbes inactive by breaking bonds between the Thymine and Adenine pair and “glues” two adjacent Thymine nucleotides together. This process is irreversible and stops the microbes from undergoing mitosis and causing harm.
Germicidal UV air disinfection has proven efficacy against the transmission of measles, probably one of the most infectious diseases known to mankind, as well as Tuberculosis, SARS-CoV, SARS-CoV-2 (Covid-19), MERS-CoV, Influenza A, “ Swine Flu” and “Bird Flu”, as well as many other bacteria and viruses. These have all been successfully inactivated by UV Air Treatment solutions in HVAC and via Upper Room
Emitters, both in the labs, and in practical applications over decades. (see our supplementary references document.)
Coronaviruses, are easily inactivated by calculated UVC exposure. (Source) 3 The data from 2004 and 2020, shows how easily Coronavirus is inactivated by UV-C technology. “The
survival ability of SARS coronavirus in human specimens and environments seems to be relatively strong. Ventilation and UV irradiation can efficiently eliminate the viral infectivity” (Source) 4
Why choose PP-L?
Our team of Chartered Engineers, Scientists, and Medical experts know your environment and your risks, and we help organizations go beyond government guidance and indeed, beyond Covid-19, to make your operations even safer, infection resilient and more normal, once again for whatever may come in the future. The Engineering & Technologies that we use to prevent and control infection are safe, proven and reliable solutions backed decades of scientific research, engineering, and practical real-world application against all known pathogens.
In fact, we are the only government-listed medical services provider of germicidal UV-C health technology in the UK. We are also an NHS Approved Supplier.
We follow the science to help you protect your people and your business better, both now and for the future.
To see how we can help your workplace, contact us today:
enquiries@ppl-biosafety.com
Bibliography
- The effect of temperature on persistence of SARS-CoV-2 on common surfaces (https://virologyj.biomedcentral.com/articles/10.1186/s12985-020-01418-7)
2. Upper-room ultraviolet air disinfection might help to reduce COVID-19 transmission in buildings: a feasibility study (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7566754/)
Why choose PP-L ?
Our team of chartered engineers, scientists, and medical experts know your environment and your risks, and we help organizations like yourselves go beyond the guidance of governments, to make your inside environments extremely COVID-secure. Our hybrid capability enables us to re-engineer infection and significantly reduce the risk. The technologies and tools that we use to improve COVID security are trusted and reliable solutions that build on decades of scientific data, engineering, practical real-world application and experince. In fact, we are the only government-listed medical services provider of germicidal UV-C health technology in the UK, and we always follow the science.
To see how we can help your workplace, contact us today:
0800 471 4871
Bibliography
(1) World Health Organization. Severe Acute Respiratory Syndrome (SARS) https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=tab_1.
(2) CDC. SARS https://www.cdc.gov/sars/about/fs-sars.html.
(3) Olsen, S. J.; Chang, H.-L.; Cheung, T. Y.-Y.; Tang, A. F.-Y.; Fisk, T. L.; Ooi, S. P.-L.; Kuo, H.-W.; Jiang, D. D.-S.; Chen, K.-T.; Lando, J.; Hsu, K.-H.; Chen, T.-J.; Dowell, S. F. Transmission of the Severe Acute Respiratory Syndrome on Aircraft. New England Journal of Medicine 2003, 349 (25), 2416–2422. https://doi.org/10.1056/nejmoa031349.
(4) HO, P.-L.; TANG, X.-P.; SETO, W.-H. SARS: Hospital Infection Control and Admission Strategies. Respirology 2003, 8 (s1), S41–S45. https://doi.org/10.1046/j.1440-1843.2003.00523.x.
(5) Wong, T.; Wallington, T.; McDonald, L. C.; Abbas, Z.; Christian, M.; Low, D. E.; Gravel, D.; Ofner, M.; Mederski, B.; Berger, L.; Hansen, L.; Harrison, C.; King, A.; Yaffe, B.; Tam, T. Late Recognition of SARS in Nosocomial Outbreak, Toronto. Emerging Infectious Diseases 2005, 11 (2), 322–325. https://doi.org/10.3201/eid1102.040607.
(6) Darnell, M. E. R.; Subbarao, K.; Feinstone, S. M.; Taylor, D. R. Inactivation of the Coronavirus That Induces Severe Acute Respiratory Syndrome, SARS-CoV. Journal of Virological Methods 2004, 121 (1), 85–91. https://doi.org/10.1016/j.jviromet.2004.06.006.
(7) Duan, S.-M.; Zhao, X.-S.; Wen, R.-F.; Huang, J.-J.; Pi, G.-H.; Zhang, S.-X.; Han, J.; Bi, S.-L.; Ruan, L.; Dong, X.-P.; SARS Research Team. Stability of SARS Coronavirus in Human Specimens and Environment and Its Sensitivity to Heating and UV Irradiation. Biomedical and environmental sciences: BES 2003, 16 (3), 246–255.

Leave a Reply